pH Levels in Carpet Cleaning – And Why It Matters More Than You Think

pH levels in carpet cleaning determine how a cleaning solution reacts with carpet fibers, dyes, and the soils trapped inside them. When the pH is mismatched to the carpet or the stain, the result can be color change, residue buildup, or gradual fiber damage. Proper pH control removes soil while preserving appearance and lifespan.
What pH Means in Carpet Cleaning
In carpet cleaning, pH refers to how acidic or alkaline a cleaning solution is as it interacts with carpet fibers and dyes, not its value in isolation. That interaction shapes how soils release, how fibers respond, and if colors remain stable during and after cleaning.
Unlike general household cleaning, carpets contain dyed fibers with specific tolerance ranges. A solution that works on one carpet can stress another because pH influences fiber swelling, dye movement, and residue behavior. This is why pH in carpet cleaning is best understood as a controlled interaction between chemistry, material, and soil, rather than a single “safe” number.

Acidic vs Alkaline Cleaners: How Each Interacts With Carpets
Acidic and alkaline cleaners do not work on the same problems, and they do not behave the same way once they contact carpet fibers. Their effectiveness, and their risk, comes from how each type of chemistry reacts with soils, dyes, and fiber structure.
Knowing this difference prevents accidental damage and explains why using the “wrong” cleaner can create long term issues even when a carpet looks clean at first.
Acidic Carpet Cleaners: When They Help and When They Harm
Acidic carpet cleaners are most often used to address mineral related issues. They can help correct browning, remove alkaline residues left behind from previous cleanings, and stabilize dyes on sensitive fibers.
Wool and other protein based fibers respond strongly to acidic conditions. In controlled situations, mild acidity can help restore balance after alkaline exposure. Outside that narrow window, acid can weaken fibers or disrupt dye stability. Over application or extended dwell time increases the risk of color shift rather than preventing it.
Acidic chemistry is corrective, not general purpose. Its value depends on accurate diagnosis of the residue or discoloration being addressed.
Alkaline Carpet Cleaners: Effective Soil Removal With Hidden Risks
Alkaline carpet cleaners excel at breaking down oily and greasy soils. Their chemistry supports emulsification, allowing bonded soils to release from fibers during cleaning.
That same strength creates risk. On many carpets, especially nylon and other synthetic fibers, excess alkalinity can leave detergent residue behind. Residue changes how fibers attract soil, leading to faster darkening in traffic areas after cleaning. Repeated exposure also places cumulative stress on fibers, reducing resilience over time.
Alkaline cleaners are effective only when their strength is matched to the soil load and followed by proper rinsing and balance restoration.

Fiber Tolerance: Why the Same pH Works on One Carpet and Damages Another
Carpet fibers are not chemically equal. Each fiber type reacts differently to acidic and alkaline conditions, which is why a cleaning solution that performs well on one carpet can cause visible or delayed damage on another. Fiber tolerance defines how much chemical variation a carpet can withstand before dyes shift, texture changes, or strength is reduced.
Ignoring fiber tolerance turns pH from a cleaning tool into a stressor.
Wool and Specialty Carpet Fibers
Wool and other specialty fibers are protein based, giving them a narrow pH tolerance. These fibers react quickly to chemical changes, especially alkalinity, which can disrupt dye stability and weaken the fiber structure over time.
Even moderate pH imbalance can cause dye movement rather than immediate fading. This often appears as uneven color or subtle lightening after the carpet dries. Because the damage accumulates gradually, the connection to pH exposure is frequently missed until it becomes irreversible.
For wool and specialty carpets, pH control is not about strength, it is about precision.
Nylon and Common Residential Carpets
Nylon carpets have a broader pH tolerance than natural fibers, which is why they are common in residential settings. This flexibility allows alkaline cleaners to remove oily soils effectively when used correctly.
The risk with nylon is not immediate damage but residue related stress. Excess alkalinity or incomplete rinsing leaves detergent behind, altering how fibers attract soil. Over time, this leads to rapid resoiling, traffic lane darkening, and a dull appearance despite frequent cleaning.
Nylon tolerates more chemical variation, but it does not tolerate imbalance indefinitely.
Commercial Carpet Systems
Commercial carpet systems are designed for durability and heavy traffic, giving them a higher tolerance for pH variation. Note, this tolerance assumes proper neutralization after cleaning.
Without balance restoration, repeated exposure to alkaline chemistry accelerates fiber fatigue and appearance loss. In commercial environments, the consequences show up as uneven wear patterns and shortened replacement cycles rather than sudden damage.
Higher tolerance reduces risk, it does not eliminate it.
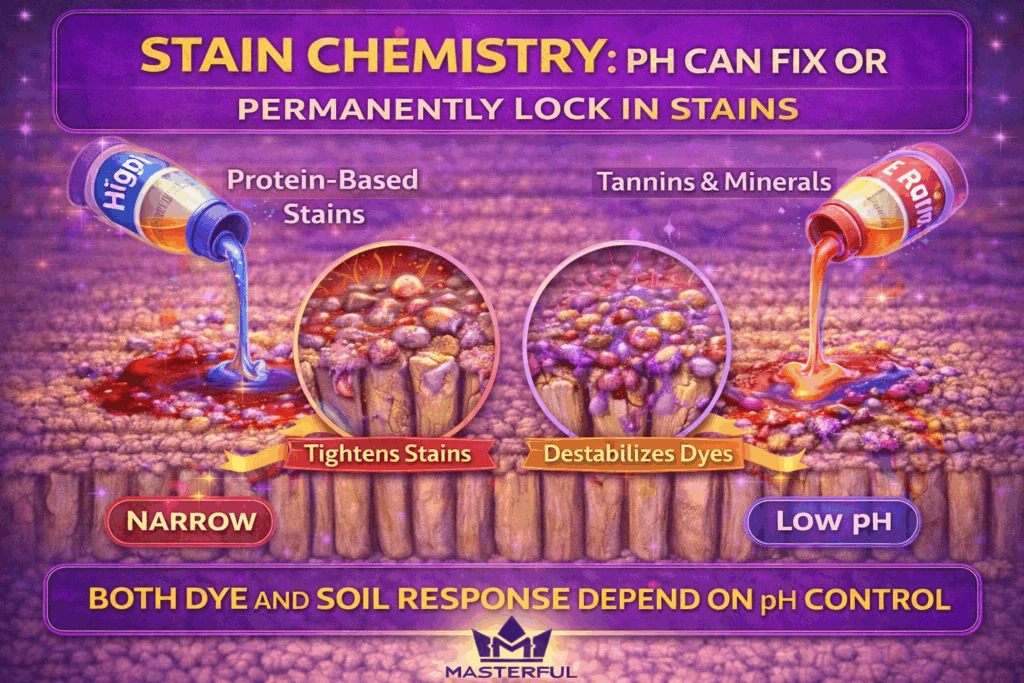
Stain Chemistry: How pH Can Lock Stains In Permanently
Stains are not just visible spills, they are chemical compounds bonded to carpet fibers. pH determines if those bonds loosen for removal or tighten into the fiber structure. When the chemistry is mismatched, cleaning can fix a stain in place instead of removing it.
This is why timing and pH selection matter more than agitation or repetition.
Protein Based Stains and Alkalinity
Protein based stains, such as blood, food residues, and some pet-related soils, react strongly to alkaline conditions. High alkalinity can cause these stains to coagulate, tightening their bond to the fiber and making later removal far more difficult.
Once set, the stain may lighten temporarily during cleaning and then reappear as the carpet dries. At that point, additional alkaline exposure increases the risk of dye instability rather than improving results.
Tannins, Minerals, and Acidic Reactions
Tannin based stains from beverages and plant matter respond differently. Acidic chemistry can help release these compounds, but only within a controlled range. Excess acidity can destabilize dyes or alter fiber texture, especially on sensitive carpets.
Mineral content in water or previous residues further complicates this process. When acids interact with minerals already present in the carpet, discoloration can intensify instead of clearing.
Mixed Soils and Sequencing Logic
Many real world stains are mixed, combining proteins, oils, and pigments. Treating these stains with a single pH approach often fails because one component reacts while another locks in.
Professional stain removal relies on sequencing, addressing each component in the correct order while monitoring pH throughout the process. Without that control, repeated cleaning attempts increase chemical stress without improving results.
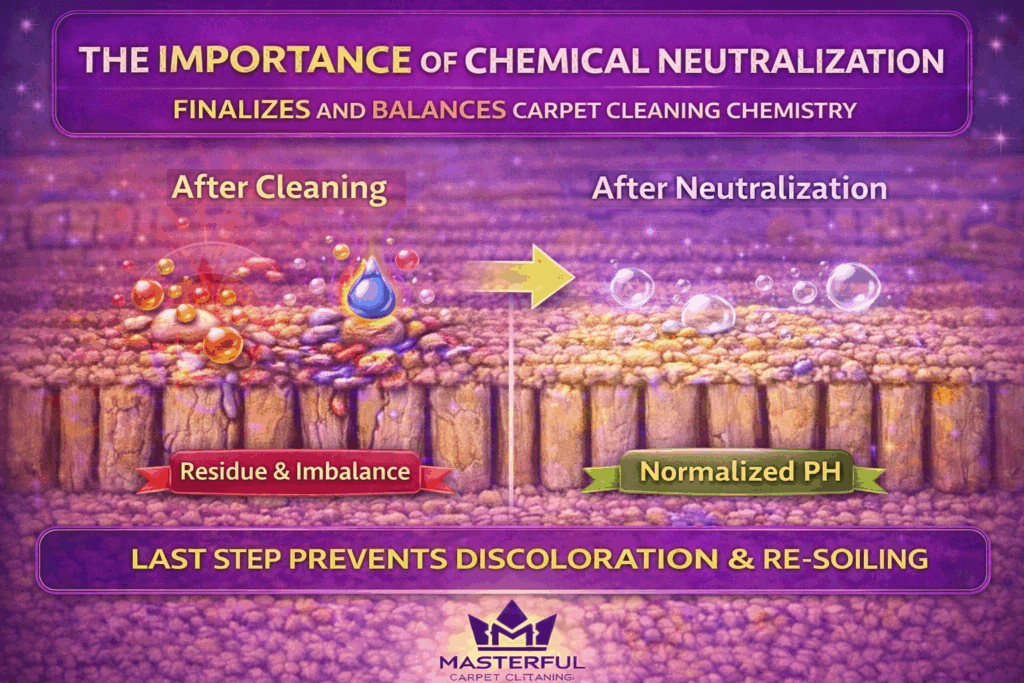
Neutralization: The Step That Finishes Carpet Cleaning Chemistry
Neutralization is the process that restores balance after cleaning chemistry has done its job. Without it, carpet cleaning remains chemically incomplete, even if the surface looks clean when wet.
During cleaning, acidic or alkaline solutions alter how soils release from fibers. Neutralization brings the carpet back toward a stable state, reducing chemical stress on fibers and helping dyes remain fixed once the carpet dries. This step is not corrective, it is preventative.
When neutralization is skipped or rushed, residual chemistry stays active inside the carpet. That leftover imbalance is a primary contributor to residue re-soiling, delayed discoloration, and texture changes that appear days or weeks after cleaning.
How Rinse Extraction Restores Balance
Rinse extraction removes loosened soil while flushing away leftover chemistry. This process reduces detergent residue and helps normalize the carpet’s pH instead of allowing it to drift toward acidity or alkalinity.
Proper rinsing is especially important after alkaline cleaning, where residues can otherwise remain sticky at the fiber level. Balanced carpets release soil more slowly, dry more evenly, and maintain appearance longer between cleanings.
Neutralization does not undo cleaning, it completes it.
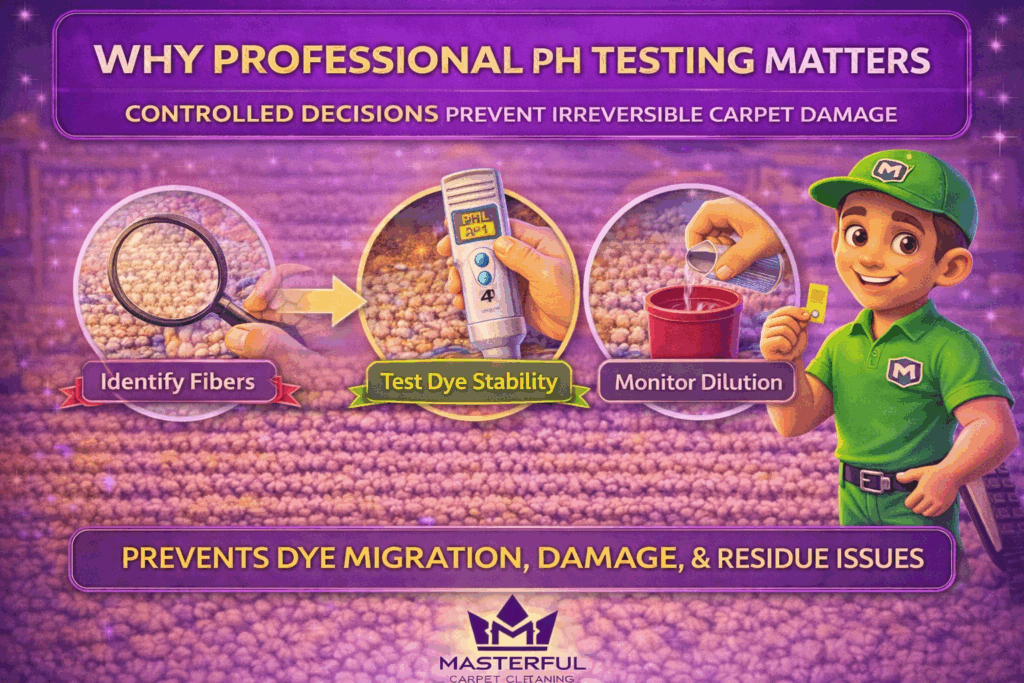
Why Professional pH Testing Prevents Irreversible Carpet Damage
Professional pH testing turns carpet cleaning from trial and error into controlled decision making. Before cleaning begins, testing identifies how a carpet’s fibers and dyes are likely to react to chemistry, allowing the cleaning process to be adjusted before damage occurs.
This step is preventive, not corrective. Once dye instability, fiber weakening, or chemical residue issues appear, options become limited. Testing reduces that risk by matching chemistry to material tolerance instead of assuming a one size approach.
Fiber Identification Before Chemistry Is Applied
Not all carpets are labeled accurately, and many older or specialty carpets lack clear documentation. Professional testing confirms fiber type before any cleaning solution is introduced.
This matters because fiber composition determines acceptable pH exposure. Identifying whether a carpet is protein-based, synthetic, or blended ensures the chemistry used stays within safe limits from the start.
Dye Stability and Controlled Dilution
Dye stability testing checks whether color is likely to migrate when exposed to moisture and chemistry. Even small pH shifts can trigger movement on sensitive carpets.
Controlled dilution follows from this information. Rather than using stronger chemistry “just in case,” professionals adjust concentration to achieve soil removal while minimizing chemical stress. This balance protects appearance without sacrificing effectiveness.
Neutralization as Part of the Process, Not an Afterthought
Professional testing does not stop once cleaning begins. Monitoring pH throughout the process means neutralization restores balance instead of guessing that rinsing alone is enough.
This ongoing control is what prevents delayed discoloration, residue resoiling, and gradual fiber fatigue that often appear after untested cleaning attempts.
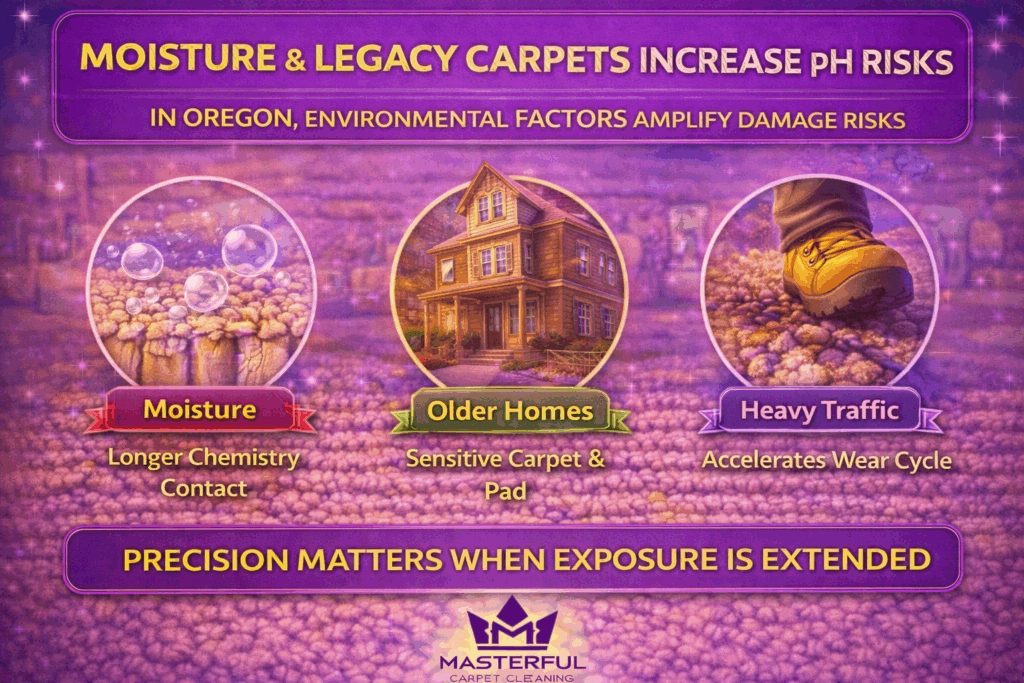
Environmental Factors in Oregon That Increase pH Risk
Environmental conditions influence how long cleaning chemistry stays active inside carpet fibers. In Oregon, moisture levels and building characteristics can amplify the effects of pH imbalance, making precision more important than in drier or newer environments.
Higher ambient humidity slows evaporation. When carpets stay damp longer, residual chemistry has more time to interact with fibers and dyes. This extended contact increases the likelihood of residue resoiling or delayed discoloration if balance is not fully restored.
Older homes introduce additional variables. Historic construction often includes legacy carpet installations, specialty fibers, or padding materials that react differently to moisture and chemistry. These conditions narrow the margin for error and increase the value of controlled pH use rather than generalized cleaning approaches.
In commercial settings, sustained foot traffic compounds the issue. Moisture combined with residual alkalinity accelerates soil attraction, causing traffic lanes to darken faster after cleaning. What appears to be normal wear is often a chemistry imbalance revealed by environmental stress.
Oregon’s conditions do not change how pH works, but they increase the consequences when it is ignored.

When DIY Cleaning Stops Being Safe
DIY carpet cleaning works only within a narrow margin of chemical tolerance. Once that margin is crossed, repeated attempts often increase damage instead of fixing the problem. Knowing when to stop is what prevents small issues from becoming permanent.
One warning sign is recurring stains or darkening. When a cleaned area looks acceptable while damp but darkens again as it dries, residue or pH imbalance is usually involved. Additional cleaning without correcting the chemistry compounds the issue rather than resolving it.
Color change or texture shift is another indicator. Subtle lightening, uneven tone, or stiffness after drying suggests fiber stress or dye movement. These changes are not surface-level problems and cannot be reversed by stronger cleaners or more agitation.
DIY cleaning also becomes unsafe when fiber type is unknown. Vintage carpets, heirloom rugs, and specialty installations often have narrow tolerance ranges that are not visually obvious. Applying generalized chemistry without testing increases the risk of irreversible damage.
At this stage, the safest option is not a different product, it is stopping further chemical exposure.
FAQ About pH Levels in Carpet Cleaning
What pH is considered safe for carpet cleaning?
There is no single pH that is safe for all carpets. Acceptable pH depends on fiber type, dye stability, and soil conditions. Some carpets tolerate mild alkalinity, while others require near neutral conditions to avoid damage.
Can the wrong pH permanently damage carpet fibers?
Yes. Repeated exposure to the wrong pH can weaken fibers, cause dye movement, and alter texture over time. This damage often develops gradually and may not be visible until it becomes irreversible.
Is acidic or alkaline cleaner better for carpet stains?
Neither is universally better. Alkaline cleaners work well on oily soils, while acidic cleaners address mineral and tannin related issues. Using the wrong pH for a specific stain can lock it into the carpet instead of removing it.
Why do carpets look dirty again after cleaning?
This usually happens because detergent residue remains in the carpet. Residual alkalinity changes how fibers attract soil, causing faster resoiling even when the carpet was thoroughly cleaned.
Do professional carpet cleaners test pH?
Yes. Professional cleaners test pH to match cleaning chemistry to fiber tolerance and dye stability. This testing helps prevent discoloration, residue problems, and long term fiber damage.
As the Co-Owner of Masterful, Randy has been providing quality cleaning services to the Salem and Portland areas of Oregon for many years. He has built a reputation for excellence in the industry. His team take prides in using the latest cleaning techniques and technologies to deliver exceptional results every time. Author






